EXPO 2025
Special Page
Event / Presentation
CHITOSE presented at Biologics Manufacturing Korea 2024, Korea’s premier bioprocessing-focused conference, held on August 13th and 14th in Incheon, South Korea. We extend our heartfelt gratitude to FUJIFILM Life Sciences Korea Co., Ltd. for their generous support and for providing us with this opportunity.
The conference attracted over 150 attending organizations, and covered themes such as high-throughput cell line development, cell culture, chromatography, analytics, quality assurance, automation, Bioprocessing 4.0, and digitalization.Kevin Montagne, BioEngineer at CHITOSE, delivered an individual presentation titled “Fast growing CHO-MK cells successfully produced 10 g/L of antibodies in 5 days”. CHO-MK* is a novel cell line established from the primary cell culture of Chinese hamster ovary tissue, designed for industrial applications in biopharmaceutical production. CHO-MK cells can accelerate the manufacturing process for recombinant therapeutic antibodies from the early stages of cell line development.
The presentation highlighted CHO-MK cells’ significant potential to reduce manufacturing time and increase access to biologics medication. The earlier findings suggested the remarkable efficiency of CHO-MK host cells, which have a doubling time of less than 10 hours and can achieve a productivity of 10 g/L in just 5 days using a bioreactor.
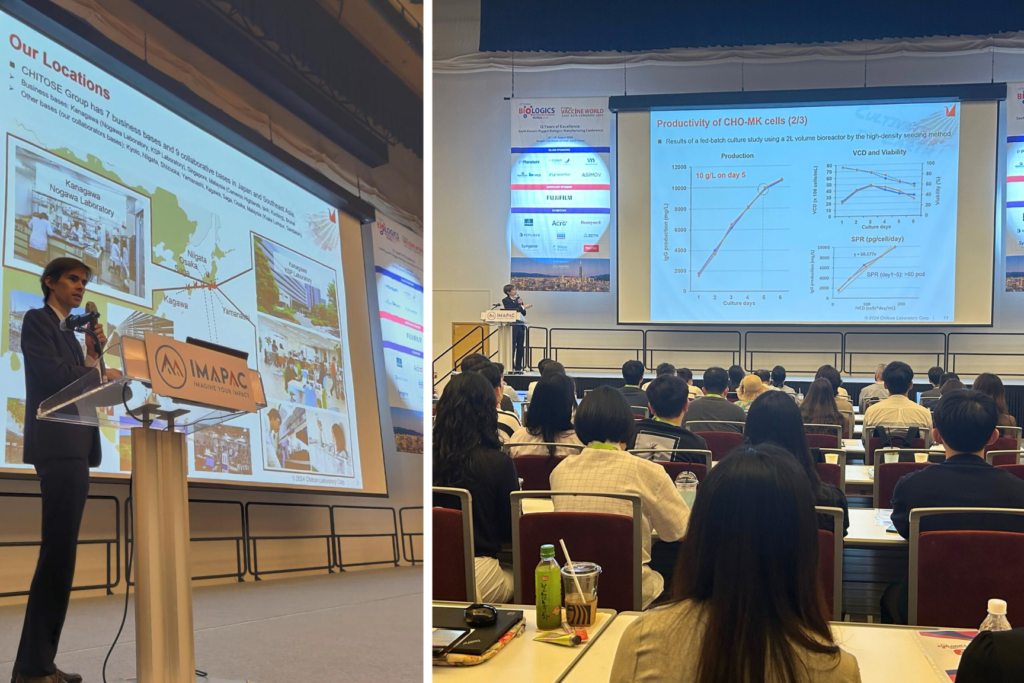
The presentation attracted around 150 attendees and sparked a wide range of questions, covering both technical and business aspects. In addition, a poster presenting information about the CHO-MK cell line was showcased at the FUJIFILM Life Sciences Korea booth, highlighting the ongoing cooperation between the two companies.
Participating in Biologics Manufacturing Korea 2024 was a valuable opportunity for CHITOSE to highlight recent developments and connect with potential clients and partners.
Event overview:
Event name: Biologics Manufacturing Korea 2024
Date: August 13th (Tuesday) – August 14th (Wednesday), 2024
Venue: Songdo Convensia, Incheon, South Korea
Stable Cell Line Development Service for Biopharmaceutical Production
Using CHO-MK and the proprietary CS™ CHO vector expression system, CHITOSE provides a high-speed Cell Line Development service (Speedy CS™) in only 12 weeks capable of generating pre-master cell banks (PCB) that highly express recombinant proteins of interest.
Related Information:
Stable Cell Line Development Service for Biopharmaceutical Production
Research Paper Published on CHO-MK: Novel Host Cell Line for Biopharmaceutical Manufacturing
[External] Establishment of a novel cell line, CHO-MK, derived from Chinese hamster ovary tissues for biologics manufacturing
[External] Biologics Manufacturing Korea 2024