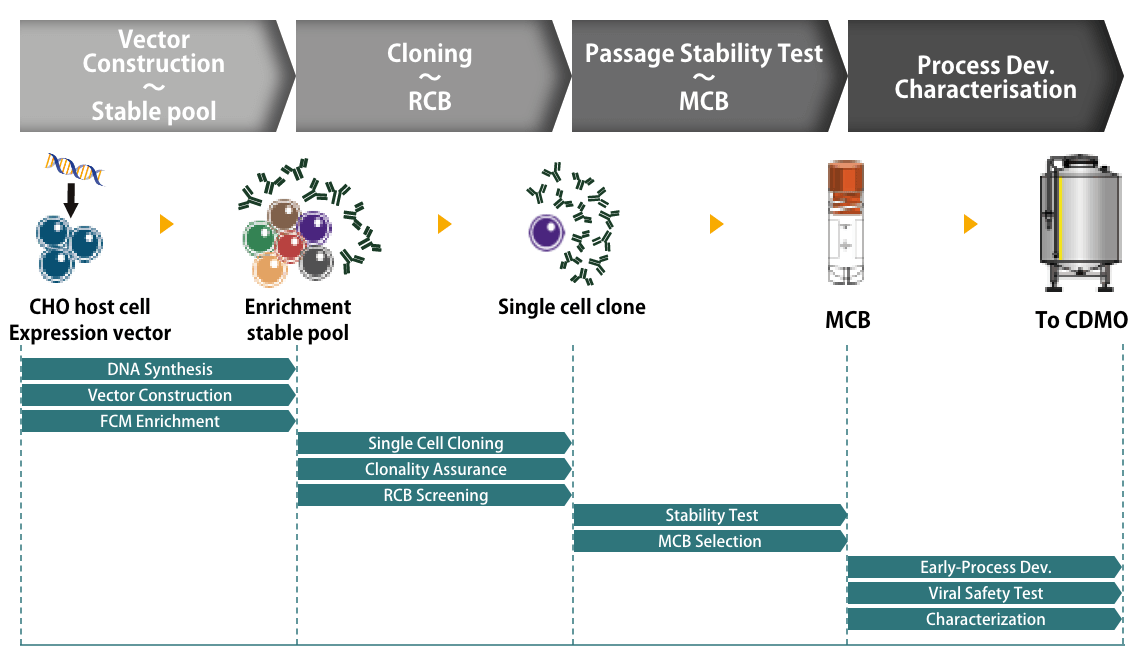
CHO cells were first developed by Dr. Theodore Puck in 1957 for research purposes (Puck, 1957, Puck et al., 1958), resulting in limited productivity and the need to improve their performance. Therefore, we established our original CHO host cells from Chinese hamster ovaries, CHO-MK cells, that are compatible with industrial purposes. Having been screened and selected for high growth and high productivity, CHO-MK cells were successfully developed and are capable of meeting industrial needs such as for large-scale biopharmaceutical manufacturing (Masuda et al., 2024).
References
Masuda K, Kubota M, Nakazawa Y, Iwama C, Watanabe K, Ishikawa N, Tanabe Y, Kono S, Tanemura H, Takahashi S, Makino T, Okumura T, Horiuchi T, Nonaka K, Murakami S, Kamihara M, Omasa T. Establishment of a novel cell line, CHO-MK, derived from Chinese hamster ovary tissues for biologics manufacturing. J. Biosci.Bioeng., 2024.
https://doi.org/10.1016/j.jbiosc.2024.02.005
Puck TT, Cieciura SJ, Robinson A. Genetics of somatic mammalian cells. III. Long-term cultivation of euploid cells from human and animal subjects. J Exp Med 1958, 108:945–956.
https://doi.org/10.1084/jem.108.6.945 (Open access).
Puck TT. The genetics of somatic mammalian cells. Adv Biol Med Phys 1957, 5:75-101.
https://doi.org/10.1016/b978-1-4832-3111-2.50006-7