EXPO 2025
Special Page
Event / Presentation
From October 20 to 22, 2025, CHITOSE participated in BPI Asia, one of the region’s premier bioprocessing conferences, held at the Westin Miyako in Kyoto, Japan. A key highlight was our co-presentation with Waters, which demonstrated how our collaboration can strengthen CHITOSE’s cell line development platform.
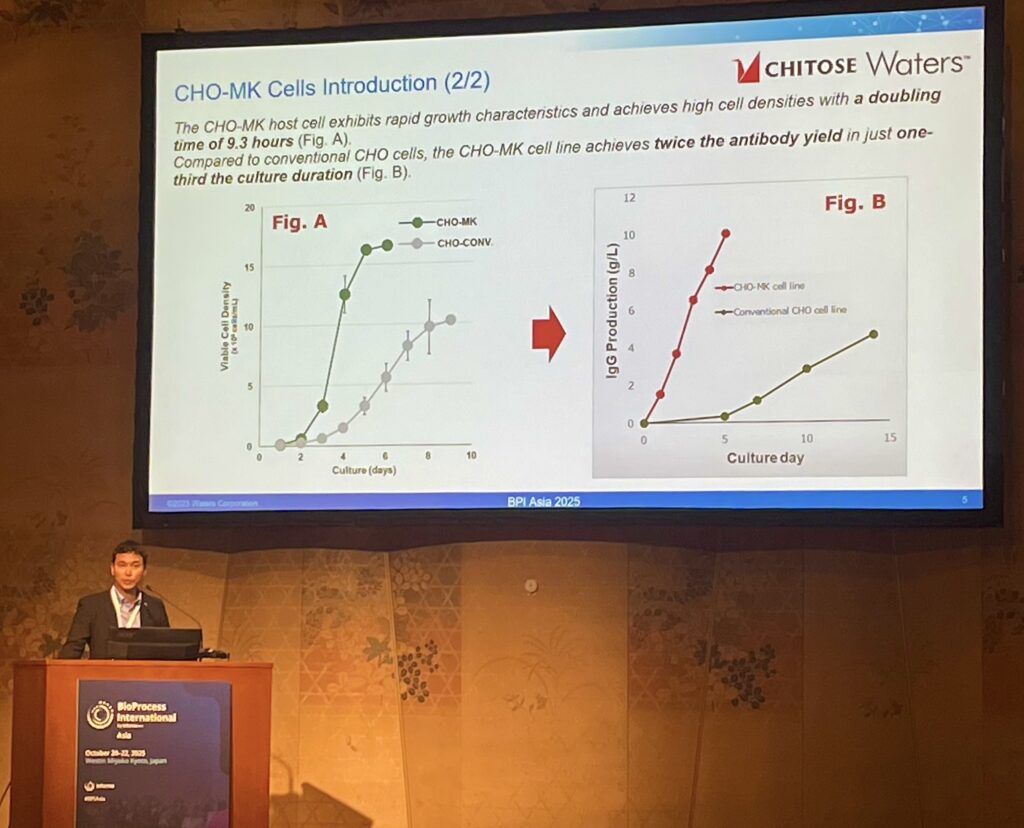
The presentation illustrated how our combined solution addresses a critical industrial constraint during early-stage process development. While CHITOSE’s high-performance CHO cells can achieve high production titers rapidly, conventional LC-MS analytics used for assessing critical product quality attributes (PQAs) of upstream cell culture bioreactor samples can take between 4-6 weeks.
This collaborative case study showed that by integrating Waters’ BioAccordTM in line with our high-throughput CHO cell culture process, turnaround time for LC-MS analytics can be shortened to days. This synergy highlights the potential for partners using our cell line development platform to gain deep, early-stage data on critical quality attributes (CQAs), allowing for significant de-risking of the scale-up process.
Event Overview
Event name: BPI Asia 2025
Date and time: Monday, October 20th – Wednesday, October 22nd, 2025
Venue: Westin Miyako (Kyoto, Japan)
Related Information:
[External] Bio Process International Official Website

The CHITOSE and Waters teams after their joint presentation at BPI Asia 2025

Terence Teo (Biologics Market Development Manager, APAC, Waters Corporation) presents the collaborative case study data
Dr. Riztyan Ian (Senior Manager/Biopharmaceutical, Gene Therapy Unit) discusses CHITOSE’s high-performance CHO cell platform